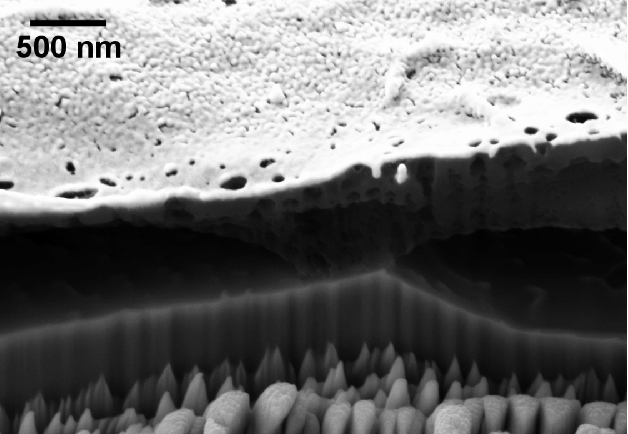
INTRODUCTION TO THE PICKLING PROCESS OF TITANIUM
Pickling is a critical surface treatment process widely employed in the manufacturing of titanium and its alloys. The primary objective of pickling is to remove oxide scales, surface contaminants, and other undesirable layers that form during high-temperature processing steps such as forging, annealing, or welding . This process not only enhances the surface finish but also prepares the titanium surface for subsequent operations, such as coating or bonding.[1]
In the pickling process for titanium, a mixture of hydrofluoric acid (HF) and nitric acid (HNO₃) is typically used. The roles of these acids are crucial:
⦁ Hydrofluoric Acid (HF): HF is a highly aggressive agent that dissolves titanium oxide (TiO₂) and other surface impurities. Its action is essential for breaking down the tough oxide layer that naturally forms on titanium surfaces due to its strong affinity for oxygen .
⦁ Nitric Acid (HNO₃): HNO₃ acts primarily as a passivating agent, controlling the reactivity of HF to prevent excessive dissolution of the titanium substrate. It also reduces the risk of hydrogen absorption, which can lead to embrittlement .[2]
The acid ratio between HF and HNO₃ is a key factor in determining the effectiveness and outcome of the pickling process. An optimal balance ensures efficient oxide removal while preserving the underlying metal’s structural integrity.
Understanding the Alpha (α) Phase of Titanium
Titanium alloys, such as commercially pure titanium and α-β alloys, predominantly contain the α-phase, characterized by a hexagonal close-packed (HCP) crystal structure. The α-phase is stable at room temperature and remains stable up to certain elevated temperatures, depending on the alloy composition .
The α-phase is distinguished by its excellent mechanical properties, including:
⦁ High Strength and Ductility: The HCP structure of the α-phase provides a good balance of strength and ductility, which is crucial for applications requiring high performance under mechanical stress .
⦁ Corrosion Resistance: The α-phase contributes significantly to the corrosion resistance of titanium alloys, making them suitable for use in highly corrosive environments, such as marine and chemical processing industries.
⦁ Creep Resistance: The stability of the α-phase at elevated temperatures ensures that titanium alloys maintain their mechanical properties over prolonged periods, making them ideal for high-temperature applications such as aerospace components .[3]
Effects of the Alpha Phase on Titanium Properties
The α-phase layer on the surface of titanium plays a pivotal role in determining the overall performance of the metal. The presence and characteristics of this layer influence several key properties:
⦁ Corrosion Resistance: The α-phase is more resistant to corrosion compared to the β-phase due to its HCP structure, which is less susceptible to chemical attack. This makes the α-phase crucial for applications where long-term exposure to corrosive environments is expected .
⦁ Surface Hardness and Wear Resistance: The α-phase contributes to the surface hardness of titanium alloys, which enhances their wear resistance. This is particularly important in applications where the material is subject to friction and abrasion .
⦁ Weldability: Titanium alloys with a stable α-phase exhibit better weldability, with reduced risks of cracking and other defects. The stability of the α-phase during welding processes helps maintain the integrity of the joint .[4]
Influence of Acid Ratio in Pickling on the Alpha Phase Layer
The acid ratio in the pickling process not only affects the surface finish and corrosion resistance but also directly impacts the thickness, integrity, and composition of the α-phase layer on titanium. Understanding how the α-phase changes with varying acid ratios is crucial for optimizing the pickling process.
⦁ Material Removal and Alpha Phase Thickness:
High HF Concentration: Increasing the concentration of HF in the pickling solution enhances the chemical aggressiveness of the treatment. This leads to more significant material removal from the surface, including the α-phase layer. As the HF concentration rises, the thickness of the α-phase decreases, potentially exposing the underlying β-phase, which is less resistant to corrosion and mechanical wear. This reduction in α-phase thickness can compromise the overall performance of the titanium, especially in applications requiring high corrosion resistance and surface integrity .[5]
High HNO₃ Concentration: Conversely, a higher concentration of HNO₃ tends to reduce the rate of material removal. Nitric acid promotes the formation of a stable oxide layer that protects the α-phase from excessive dissolution. As a result, the α-phase layer remains relatively thicker and more intact compared to treatments with higher HF concentrations. This preservation of the α-phase enhances the corrosion resistance and mechanical properties of the titanium surface. However, if the HNO₃ concentration is too high, the pickling process may become less effective at removing surface contaminants and oxides, potentially leaving an incomplete or unevenly etched surface.[6]
⦁ Alteration of Microstructure:
Microstructural Changes with High HF: A higher HF concentration can lead to more aggressive etching, not only reducing the α-phase thickness but also potentially altering its microstructure. The excessive removal of material can cause micro-pitting and roughness, which may introduce defects into the α-phase layer. These microstructural changes can negatively impact the mechanical properties, such as fatigue resistance and ductility .
Preservation with High HNO₃: With higher levels of HNO₃, the α-phase layer is better preserved in its original state. The microstructure remains more stable, which is beneficial for maintaining the material’s inherent properties. The passivating effect of nitric acid helps to protect the grain boundaries within the α-phase, reducing the risk of intergranular attack and maintaining the overall toughness and integrity of the titanium alloy.[7]
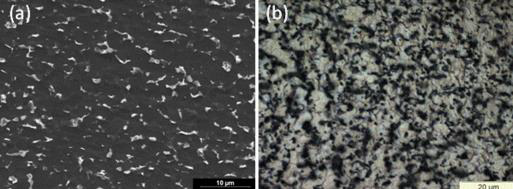
Figure 1 (a) SEM view and (b) OM view of rolled sheet microstructure, previously pickled in a mixed 4 w%HF–3 w%HNO3 solution for 10 s at ambient temperature [9]
- Oxygen and Hydrogen Profiles: The provided text explains that the oxygen profile after pickling in a 20 wt% HNO₃ and 2 wt% HF solution (for 200 seconds) shows the formation of passive layers on the surface of Ti–6Al–4V. The text further indicates that the α phase is primarily composed of titanium oxide (TiO₂), while β phase regions are associated with aluminum oxide (Al₂O₃) and vanadium oxide (V₂O₅).
- The acid ratio affects the formation of these oxides and the stability of the passive layer. Higher HNO₃ concentrations are likely to promote a thicker oxide layer on the α phase due to its oxidizing nature, whereas higher HF concentrations could lead to more aggressive etching, potentially thinning the α phase and exposing more of the β phase beneath.
- Pickling Time and Acid Ratio: The text cites studies indicating that prolonged pickling times in HF-rich solutions can increase the oxide content of the passivation layer, but this effect is dependent on the substrate composition and the presence of oxidizing agents like HNO₃. This suggests that an acid ratio with a higher concentration of HF could lead to the thinning of the α phase by dissolving the oxide layer more effectively, thereby exposing more β phase. Conversely, a higher HNO₃ concentration would stabilize the α phase by forming a protective oxide layer, reducing the exposure of the β phase.[8]
⦁ Growth of the Oxide Layer:
Oxide Layer Formation: An increase in HNO₃ concentration promotes the growth of a thin but stable oxide layer on the surface of the α-phase. This oxide layer, typically consisting of titanium dioxide (TiO₂), serves as a protective barrier against further chemical attack. While this oxide layer is beneficial for corrosion resistance, it also means that the α-phase layer underneath remains less affected by the pickling process, maintaining its original thickness and properties.
Impact on Further Processing: If the α-phase is too thin due to high HF concentrations, the surface may be less suitable for further processing, such as welding or coating, as it may not provide sufficient material strength or corrosion resistance. Conversely, a well-maintained α-phase layer, preserved by a higher HNO₃ concentration, ensures that the titanium is in optimal condition for subsequent manufacturing steps .[8]
REFERENCES
[1] ASM Handbook, Volume 5: Surface Engineering – Detailed discussion on surface treatment techniques for various materials, including titanium and its alloys.
[2] ASM Handbook, Volume 4: Heat Treating – Provides insights into the high-temperature processes leading to oxide formation on titanium and the subsequent pickling process.
[3] ASM Handbook, Volume 13A: Corrosion: Fundamentals, Testing, and Protection – Examines the impact of surface treatments on the corrosion behavior of titanium alloys.
[4] ASM Handbook, Volume 2: Properties and Selection: Nonferrous Alloys and Special-Purpose Materials – Covers the properties and phase compositions of titanium alloys, with emphasis on the α-phase.
[5] Kurt, A., et al. “The Effect of Pickling Solution Composition on the Surface Roughness and Mechanical Properties of Titanium Alloys.” Journal of Materials Engineering and Performance, vol. 22, no. 10, 2013, pp. 2814-2820.
[6] Li, Y., et al. “Effects of Hydrofluoric Acid Concentration on the Surface Morphology and Corrosion Resistance of Pickled Titanium Alloys.” Corrosion Science, vol. 89, 2014, pp. 191-199.
[7] YOU Li-mei, PENG Dong-qiang, ZHOU Lin-yan, et al. “Acid Pickling Process of Titanium Alloys and Its Investigation of Intergranular Corrosion and Pitting Corrosion.” International Conference on Manufacturing Science and Engineering (ICMSE 2015), Atlantis Press, 2015.
[8] Mishra, R.S., et al. “Role of Passivation in the Corrosion Resistance of Pickled Titanium Alloys.” Materials Science and Engineering: A, vol. 527, no. 3, 2010, pp. 726-733.
[9] VERMESSE, Eric; MABRU, Catherine; ARURAULT, Laurent. Surface integrity after pickling and anodization of Ti–6Al–4V titanium alloy. Applied Surface Science, 2013, 285: 629-637.